Frequently Answered Questions
Submit your new account form found here: New Account Form
Email a completed Talon_RFA8.0 and TA-014D Fillable Talon Chain of Custody Form
to: samplesubmission@talonanalytical.com
*Please note, that samples that are delivered by the client are for R&D purposes only.
An example of a sample order form can be found here: Talon_RFA8.0_Example
Other questions? Reach out to our team at 888-201-0957 or Samplesubmission@talonanalytical.com
We’re open for pre-scheduled sample deliveries 9 AM- 5 PM Monday-Friday.
Call to schedule your sample delivery, 888-201-0957
We accept ACH or cash payments.
Reach out to samplesubmission@talonanalytical.com for our ACH payment details if looking to complete your payment with this method.
An email will notify the client their report is ready to be viewed. COA reports are accessed through Confident LIMS
Account setup and walk-through of Confident LIMS will take place at the time of a client’s first sample submission.
Compliance Sample – cannabis product collected from a lot or batch that has been fully processed. Samples must be placed in their final packaging before or during the sampling process as intended for sale.
Research & Development – sample of any product or intermediary not intended for final sale
Process Sample (Research & Development) – cannabis product that is drawn from the process line at any stage of production prior to final packaging.
No, prior approval for lot testing is not required by the Office of Cannabis Management.
Yes, beginning July 1, 2023 final product samples that are being submitted for compliance testing are required to be sampled by an approved sampling firm licensed by the New York State Office of Cannabis Management. Contact us today to coordinate your sampling and transportation needs.
Full Panel Compliance Testing:
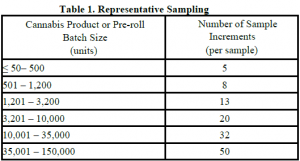
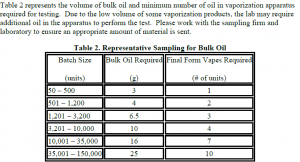
R&D Testing:
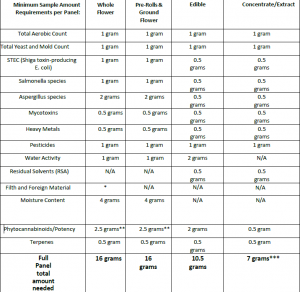
*For foreign matter testing, 30% of the sample received will be inspected. If you are sending a product for Filth & Foreign Material analysis only, please provide at least 3 grams of sample.
**If submitting samples for potency-only testing, please include 2 extra grams of flower for moisture content analysis so that cannabinoids can be reported on a dry weight basis. If not enough samples are provided, the results will be reported on an as-is basis.
***For concentrates & extracts, if products are solely in a cartridge or disposable pen, the lab may request more sample material to ensure we are able to recover enough material to complete all testing. Submitting a bulk tube with a few filled cartridges for metals testing is preferred.

